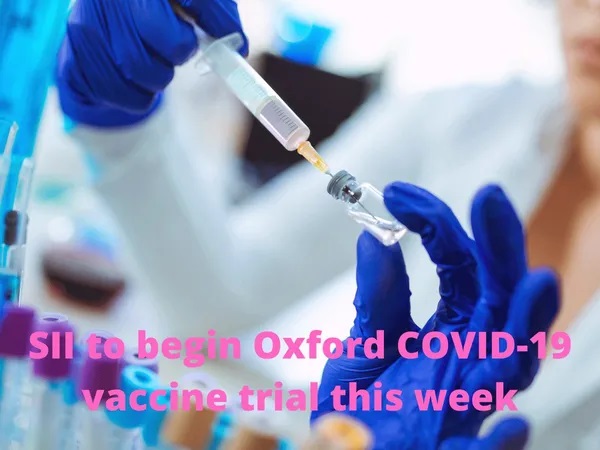
Serum Institute of India to begin phase 2 trials of Oxford COVID-19 vaccine this week
Serum Institute of India (SII) will begin the phase 2 clinical trials of a promising coronavirus vaccine developed by the University of Oxford this week.
Key Highlights
- Scientists are working at unprecedented speed to find a safe and effective vaccine against novel coronavirus infection
- The Oxford's ChAdOx1 vaccine is a chimpanzee adenovirus vaccine vector
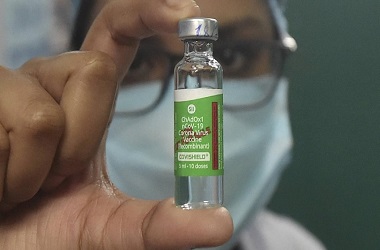
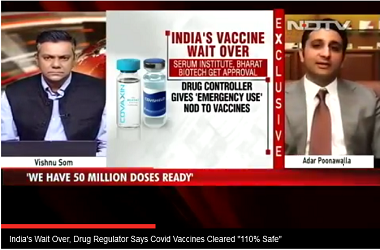
- Serum Institute of India has been permitted for conducting the phase 2 and 3 clinical trials of Covishield vaccine in India
Pune: Serum Institute of India (SII) will begin the phase 2 clinical trials of a promising coronavirus vaccine developed by the University of Oxford this week, according to reports. The Pune-based firm, which has partnered with British drug giant AstraZeneca to manufacture the Oxford’s vaccine candidate ChAdOx1, will start the trials has been granted permission by the DCGI to conduct phase 2/3 clinical trials in India.
As per reports, trials for the Oxford's COVID-19 vaccine will begin at two Mumbai's hospitals - KEM and Nair hospitals - which have begun preliminary procedures for the study. Earlier, it was revealed that SII has chosen 10 centres across the country to conduct the trials of the vaccine, to be called Covishied in India. The company had said that Covishield vaccine will be priced at Rs 224 per dose in India.
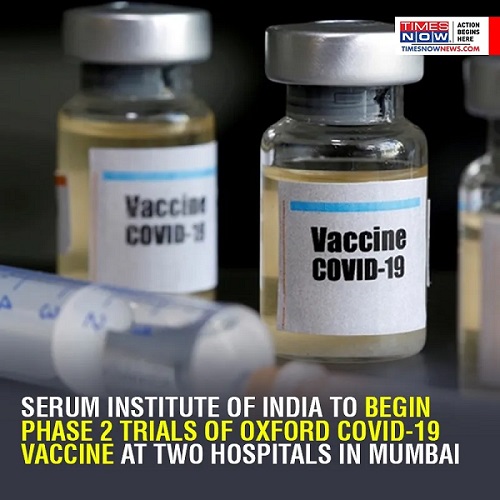
Oxford/AstraZeneca’s candidate ChAdOx1, a chimpanzee adenovirus vaccine vector, has shown encouraging results in early human trials. The results of the phase 1/2 trial published in the scientific journal, The Lancet, showed that the vaccine induces strong immune responses in both parts of the immune system and shows no early safety concerns. Researchers are currently assessing whether the vaccine will be effective in combatting COVID-19.
Source - Times Now News
ABOUT US
OUR COMPANIES
CORPORATE SOCIAL RESPONSIBILITY
© Copyright 2026. Cyrus Poonawalla Group. All rights reserved.