ICMR helping Serum Institute in conducting clinical trials for two vaccines
Country’s apex biomedical research agency is also helping Zydus Cadila for laboratory assays to detect antibody response, according to documents reviewed by Mint

ICMR made a presentation before the members of the parliamentary standing committee on home affairs on Wednesday (Photo: ANI)
The Indian Council of Medical Research (ICMR) is supporting Serum Institute of India (SII) in conducting the phase 2 and 3 clinical trials for two vaccines for covid-19, one developed by University of Oxford and the other by US-based Novavax.
Country’s apex biomedical research agency is also helping Zydus Cadila for laboratory assays to detect antibody response, according to documents reviewed by Mint. The ICMR made a presentation before the members of the parliamentary standing committee on home affairs on Wednesday.
Dr Balram Bhargava, Director-General ICMR, according to sources, told the parliament panel that the vaccine candidates developed by Oxford University handled by SII are going to enter phase-2 trials by the end of this week and about 1,700 patients have been identified at 17 centres across the country for trial.
In a clinical trial, every site has a committee which monitors whether the study meets ethical guidelines and the trial protocol, without which principal investigators of that particular site cannot go ahead.
The ICMR in its presentation informed members of the committee that Bharat Biotech’s inactivated vaccine candidate, co-developed with the National Institute of Virology and Zydus Cadila Ltd’s DNA plasmid vaccine are currently in Phase 1 clinical trials.
“The ICMR is supporting both the companies in parallel pre-clinical studies of safety, immunogenicity and efficacy in non-human primates and laboratory assays for antibody detection and immunological response assessment," the ICMR presentation document stated. Meanwhile, SII is currently also undertaking a phase 3 clinical trial of Astrazeneca plc and University of Oxford’s jointly-developed adenovirus-based covid-19 vaccine.
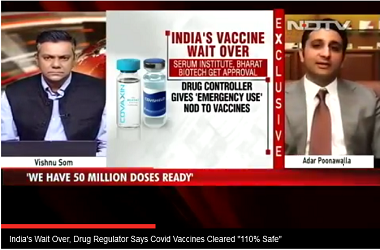
Bhargava during the meeting indicated that an emergency authorisation could be considered for covid-19 vaccines, if the government takes a decision. However, the ICMR also hinted that it will rigorously follow the ethics and safety protocols before taking any concrete decision.
“Vaccine trials needs to follow all ethics and science required for that. Vaccine trials are in process and we can speak only when some results will be available," said Dr Rajni Kant, head, research management, policy, planning and coordination division, ICMR.
The government has already said that India is not planning to follow a pre-set benchmark for selection of a covid-19 vaccine while exploring options for mass immunisation drive for the highly infectious disease. The National Expert Group on Vaccine Administration has been constituted which has been tasked with keeping logistics, financial and other support ready when a vaccine is approved, and also selecting a suitable vaccine for mass administration.
Government officials said that they are looking at various benchmarks such as cost, ease of administration and efficacy to determine which of the vaccines that are approved can be used in a mass-immunisation drive against covid-19.
The number of covid-19 cases continue to rise in the country. India on Thursday recorded 68555 covid-19 cases taking the total tally to 2849765 and the toll rose to 54467.
The ICMR has been ramping up the covid-19 testing in the country. For the first time, a record number of more than 9 lakh covid-19 tests have been conducted in a single day on Thursday. With 9,18,470 covid-19 tests done in the last 24 hours, India is poised to see an exponential increase towards its resolve of testing 10 lakh samples daily. The cumulative tests on Thursday crossed 3.25 crore (3,26,61,252), the union health ministry said.
The Tests Per Million (TPM) have seen a sharp increase to 23668. As the national average falls below 8%, there are 26 States/UTs that are reporting lower rates than the national average.
There has been a steady rise in the national network of diagnostic labs too. With 977 labs in the government sector and 517 private labs, the lab infrastructure has been enhanced to 1494 labs today, the union health ministry said.
Source - livemint
ABOUT US
OUR COMPANIES
CORPORATE SOCIAL RESPONSIBILITY
© Copyright 2026. Cyrus Poonawalla Group. All rights reserved.