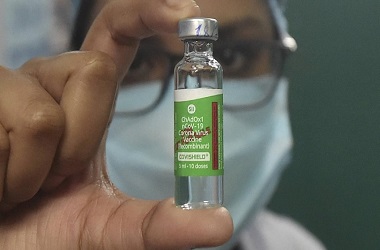
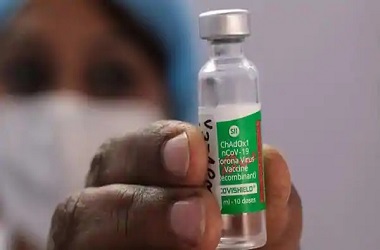
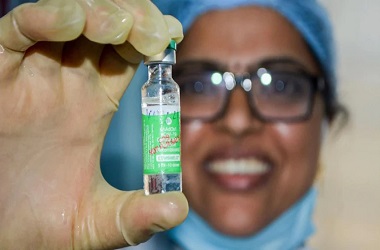
'Covishield' ready to roll out in coming weeks, says Serum Institute's Adar Poonawalla
The Pune-based vaccine major has entered into a collaboration with the University of Oxford and AstraZeneca to manufacture the vaccine The Indian regulator has granted restricted emergency use approval for Covishield and Covaxin vaccines.

NEW DELHI: Serum Institute of India (SII) on Sunday said it is ready to roll out Covishield vaccine in the country in the coming weeks after receiving approval from the Indian drug regulator.
The Pune-based vaccine major has entered into a collaboration with the University of Oxford and AstraZeneca to manufacture the vaccine.
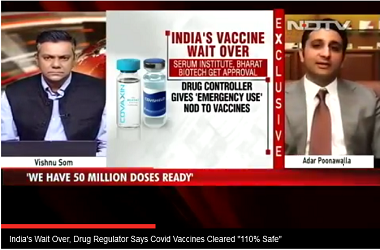
On Sunday, Drug Controller General of India (DCGI) approved Covishield and Bharat Biotech's Covaxin for restricted emergency use.
"Happy new year, everyone! All the risks @SerumInstIndia took with stockpiling the vaccine, have finally paid off. COVISHIELD, India's first COVID-19 vaccine is approved, safe, effective and ready to roll-out in the coming weeks," SII CEO Adar Poonawalla said in a tweet.
Happy new year, everyone! All the risks @SerumInstIndia took with stockpiling the vaccine, have finally paid off.
The company has already stockpiled around 50 million dosages of the vaccine and aims to produce up to 100 million dosages per month by March next year.
The approval by the DCGI was given on the basis of recommendations submitted by a COVID-19 subject expert committee (SEC) of the Central Drugs Standard Control Organisation (CDSCO).
"After adequate examination, CDSCO has decided to accept the recommendations of the Expert Committee and accordingly, vaccines of M/s Serum and M/s Bharat Biotech are being approved for restricted use in emergency situation," DCGI Dr V G Somani told a press conference.
This paves the way for the roll-out of at least two vaccines in India in the coming days.
The Oxford-AstraZeneca vaccine has already been approved by the UK government.
Besides SII and Bharat Biotech, Pfizer has also applied to the DCGI seeking emergency use authorisation for their COVID-19 vaccines
Source - The Economic Times
ABOUT US
OUR COMPANIES
CORPORATE SOCIAL RESPONSIBILITY
© Copyrights 2026. Cyrus Poonawalla Group. All rights reserved.